DTU Health Tech
Department of Health Technology
This link is for the general contact of the DTU Health Tech institute.
If you need help with the bioinformatics programs, see the "Getting Help" section below the program.
DTU Health Tech
Department of Health Technology
This link is for the general contact of the DTU Health Tech institute.
If you need help with the bioinformatics programs, see the "Getting Help" section below the program.
EasyGibbs Prediction method training server.
The training sequences can be input in the following two ways:
At any time during the wait you may enter your e-mail address and simply leave the window. Your job will continue; you will be notified by e-mail when it has terminated. The e-mail message will contain the URL under which the results are stored; they will remain on the server for 24 hours for you to collect them.
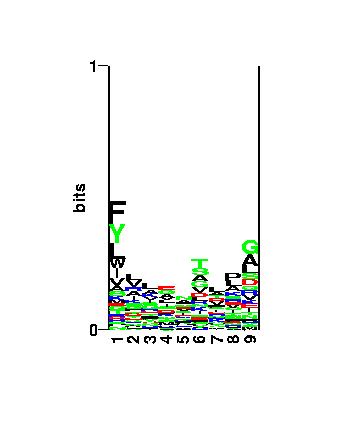
Peptide Start res Motif Prediction Assign Sequence 1 8 FWSFGSEDG 6.549 5.400 MASPGSGFWSFGSEDGSGDS 2 1 FGSEDGSGD 3.304 62.000 FGSEDGSGDSENPGRARAWC 3 6 ARAWCQVAQ 3.363 100.000 ENPGRARAWCQVAQKFTGGI 4 1 QVAQKFTGG 1.638 100.000 QVAQKFTGGIGNKLCALLYG 5 8 LYGDAEKPA 4.603 100.000 GNKLCALLYGDAEKPAESGG 6 7 ESGGSQPPR 1.463 100.000 DAEKPAESGGSQPPRAAARK 7 8 ARKAACACD 3.170 100.000 SQPPRAAARKAACACDQKPC 8 12 CSKVDVNYA -1.160 2.400 AACACDQKPCSCSKVDVNYA 9 12 LHATDLLPA 6.138 0.500 SCSKVDVNYAFLHATDLLPA 10 2 LHATDLLPA 6.138 0.200 FLHATDLLPACDGERPTLAF 11 12 QDVMNILLQ 1.590 100.000 CDGERPTLAFLQDVMNILLQ 12 11 YVVKSFDRS 4.430 0.700 LQDVMNILLQYVVKSFDRST 13 1 YVVKSFDRS 4.430 19.000 YVVKSFDRSTKVIDFHYPNE 14 5 FHYPNELLQ 6.535 5.000 KVIDFHYPNELLQEYNWELA 15 7 WELADQPQN 7.249 5.000 LLQEYNWELADQPQNLEEIL 16 11 MHCQTTLKY 5.006 100.000 DQPQNLEEILMHCQTTLKYA 17 9 YAIKTGHPR 9.089 100.000 MHCQTTLKYAIKTGHPRYFN 18 8 YFNQLSTGL 5.320 0.500 IKTGHPRYFNQLSTGLDMVG 19 12 AADWLTSTA 1.734 1.400 QLSTGLDMVGLAADWLTSTA 20 5 WLTSTANTN 4.697 41.000 LAADWLTSTANTNMFTYEIA 21 5 FTYEIAPVF 3.031 85.000 NTNMFTYEIAPVFVLLEYVT 22 4 MREIIGWPG 7.542 48.000 LKKMREIIGWPGGSGDGIFS 23 9 FSPGGAISN 0.479 80.000 PGGSGDGIFSPGGAISNMYA 24 9 YAMMIARFK 3.854 100.000 PGGAISNMYAMMIARFKMFP 25 11 EVKEKGMAA 3.955 25.000 MMIARFKMFPEVKEKGMAAL 26 4 EKGMAALPR 4.336 40.000 EVKEKGMAALPRLIAFTSEH 27 6 FTSEHSHFS 8.330 0.200 PRLIAFTSEHSHFSLKKGAA 28 3 FSLKKGAAA 5.693 100.000 SHFSLKKGAAALGIGTDSVI 29 10 ILIKCDERG 1.002 24.000 ALGIGTDSVILIKCDERGKM 30 10 MIPSDLERR -0.681 100.000 LIKCDERGKMIPSDLERRIL 31 8 RILEAKQKG 2.013 38.000 IPSDLERRILEAKQKGFVPF 32 10 FLVSATAGT 5.278 4.000 EAKQKGFVPFLVSATAGTTV 33 11 YGAFDPLLA 6.422 7.000 LVSATAGTTVYGAFDPLLAV 34 1 YGAFDPLLA 6.422 100.000 YGAFDPLLAVADICKKYKIW 35 10 WMHVDAAWG 9.248 2.700 ADICKKYKIWMHVDAAWGGG 36 1 MHVDAAWGG 1.533 43.000 MHVDAAWGGGLLMSRKHKWK 37 9 WKLSGVERA 7.137 0.800 LLMSRKHKWKLSGVERANSV 38 9 SVTWNPHKM 4.403 13.000 LSGVERANSVTWNPHKMMGV 39 5 HKMMGVPLQ 3.161 34.000 TWNPHKMMGVPLQCSALLVR 40 8 LVREEGLMQ 5.674 17.000 PLQCSALLVREEGLMQNCNQ 41 3 GLMQNCNQM 2.757 41.000 EEGLMQNCNQMHASYLFQQD 42 5 YLFQQDKHY 5.432 22.000 MHASYLFQQDKHYDLSYDTG 43 5 LSYDTGDKA 4.032 31.000 KHYDLSYDTGDKALQCGRHV 44 12 VFKLWLMWR 0.431 100.000 DKALQCGRHVDVFKLWLMWR 45 5 LWLMWRAKG 8.337 33.000 DVFKLWLMWRAKGTTGFEAH 46 7 FEAHVDKCL 2.003 100.000 AKGTTGFEAHVDKCLELAEY 47 12 YNIIKNREG 3.638 34.000 VDKCLELAEYLYNIIKNREG 48 2 YNIIKNREG 3.638 4.000 LYNIIKNREGYEMVFDGKPQ 49 2 EMVFDGKPQ 2.819 67.000 YEMVFDGKPQHTNVCFWYIP 50 7 WYIPPSLRT 5.691 0.600 HTNVCFWYIPPSLRTLEDNE 51 3 LRTLEDNEE 0.154 5.000 PSLRTLEDNEERMSRLSKVA 52 4 SRLSKVAPV 3.410 100.000 ERMSRLSKVAPVIKARMMEY 53 10 YGTTMVSYQ 2.533 10.000 PVIKARMMEYGTTMVSYQPL 54 3 TMVSYQPLG 1.012 10.000 GTTMVSYQPLGDKVNFFRMV 55 7 FRMVISNPA 11.762 0.700 GDKVNFFRMVISNPAATHQD 56 2 SNPAATHQD 3.258 65.000 ISNPAATHQDIDFLIEEIER 57 8 FLIEEIERL 3.306 20.000 ATHQDIDFLIEEIERLGQDL
If you need help regarding technical issues (e.g. errors or missing results) contact Technical Support. Please include the name of the service and version (e.g. NetPhos-4.0) and the options you have selected. If the error occurs after the job has started running, please include the JOB ID (the long code that you see while the job is running).
If you have scientific questions (e.g. how the method works or how to interpret results), contact Correspondence.
Correspondence:
Technical Support: