DTU Health Tech
Department of Health Technology
This link is for the general contact of the DTU Health Tech institute.
If you need help with the bioinformatics programs, see the "Getting Help" section below the program.
DTU Health Tech
Department of Health Technology
This link is for the general contact of the DTU Health Tech institute.
If you need help with the bioinformatics programs, see the "Getting Help" section below the program.
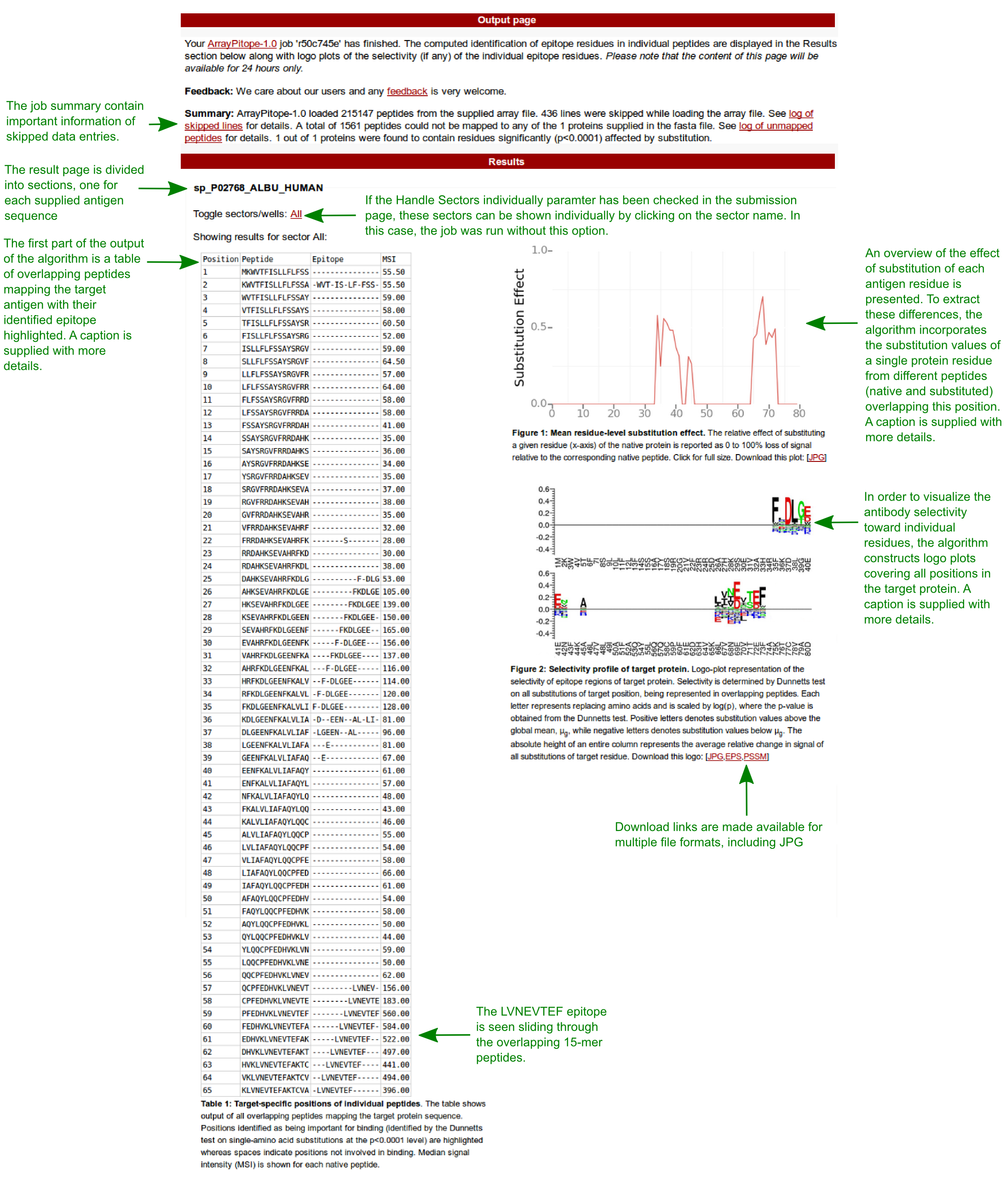
ArrayPitope performs residue-level epitope mapping of antigens of interests based on peptide microarray data.
The platform algorithm involves statistical hypothesis testing on each native-mapped synthetic peptide and produce as output a report of the antigen residues identified to be statistically significant for the preservation of binding to the antibody (i.e. high signal intensity).
ArrayPitope performs residue-level epitope mapping of antigens of interests based on peptide microarray data.
This tool has been designed with peptide microarray studies in mind that aims to characterize linear antibody epitopes using an exhaustive amino acid substitution analysis of peptides originating from target antigens.
Main reference:
NetMHCpan - MHC class I binding prediction beyond humans
Hoof I1,
Peter B3,
Sidney J3,
Pedersen LE2
Lund O1,
Buus S2,
Nielsen M1,
Immunogenetics. 2009 Jan;61(1):1-13.
1Center for Biological Sequence Analysis,
Technical University of Denmark,
DK-2800 Lyngby, Denmark
2Division of Experimental Immunology,
Institute of Medical Microbiology and Immunology,
University of Copenhagen, Denmark
3La Jolla Institute for Allergy and Immunology, San Diego, California, United States of America
Binding of peptides to major histocompatibility complex (MHC) molecules is the single most selective step in the recognition of pathogens by the cellular immune system. The human MHC genomic region (called HLA) is extremely polymorphic comprising several thousand alleles, each encoding a distinct MHC molecule. The potentially unique specificity of the majority of HLA alleles that have been identified to date remains uncharacterized. Likewise, only a limited number of chimpanzee and rhesus macaque MHC class I molecules have been characterized experimentally. Here, we present NetMHCpan-2.0, a method that generates quantitative predictions of the affinity of any peptide-MHC class I interaction. NetMHCpan-2.0 has been trained on the hitherto largest set of quantitative MHC binding data available, covering HLA-A and HLA-B, as well as chimpanzee, rhesus macaque, gorilla, and mouse MHC class I molecules. We show that the NetMHCpan-2.0 method can accurately predict binding to uncharacterized HLA molecules, including HLA-C and HLA-G. Moreover, NetMHCpan-2.0 is demonstrated to accurately predict peptide binding to chimpanzee and macaque MHC class I molecules. The power of NetMHCpan-2.0 to guide immunologists in interpreting cellular immune responses in large out-bred populations is demonstrated. Further, we used NetMHCpan-2.0 to predict potential binding peptides for the pig MHC class I molecule SLA-1*0401. Ninety-three percent of the predicted peptides were demonstrated to bind stronger than 500 nM. The high performance of NetMHCpan-2.0 for non-human primates documents the method's ability to provide broad allelic coverage also beyond human MHC molecules. The method is available at http://www.cbs.dtu.dk/services/NetMHCpan.
PMID: 19002680
If you need help regarding technical issues (e.g. errors or missing results) contact Technical Support. Please include the name of the service and version (e.g. NetPhos-4.0) and the options you have selected. If the error occurs after the job has started running, please include the JOB ID (the long code that you see while the job is running).
If you have scientific questions (e.g. how the method works or how to interpret results), contact Correspondence.
Correspondence:
Technical Support: