DTU Health Tech
Department of Health Technology
This link is for the general contact of the DTU Health Tech institute.
If you need help with the bioinformatics programs, see the "Getting Help" section below the program.
DTU Health Tech
Department of Health Technology
This link is for the general contact of the DTU Health Tech institute.
If you need help with the bioinformatics programs, see the "Getting Help" section below the program.
| AMUSER offers quick and easy design of PCR primers optimized for various USER cloning based DNA engineering |
Restrictions:
Please read the CBS access policies for information about limitations on the daily number of submissions.
Confidentiality:
The sequences are kept confidential and will be deleted
after processing.
For publication of results, please cite:
Hans Jasper Genee, Mads Tvillinggaard Bonde, Frederik Otzen Bagger, Jakob Berg Jespersen, Morten O. A. Sommer, Rasmus Wernersson, and Lars Rønn Olsen
Software-supported USER cloning strategies for site-directed mutagenesis and DNA assembly
Keywords: : DNA assembly, USER cloning, primer design, site-directed mutagenesis, point mutation, web server.
Input options Site directed mutagenesis - introduce insertions, mismatch mutations and deletions Combinatorial assembly Advanced PCR settings Salt and primer concentrations Specification of primer Tm Output options Linear Circular (default): Into a USER cassette Cassette options Basic: Predefined fusion cassettes Advanced: User designed cassettes Example of input/output data Example 1 Construction of a plasmid from 3 parts INPUT RESULTS PAGE Example 2 A point mutation and addition of a His-tag to GFP in a vector INPUT RESULTS PAGE Example 3 Construction of a small combinatorial vector library INPUT RESULTS PAGE Primer evaluation parameters Restrictions on use
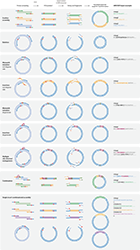
Example - Site directed mutagenesisMultiple sites can be targeted anywhere in a sequence by the introduction of more squared brackets.
Mismatch mutation
>Sequence1
ACGGACTAGCAGTCGCAGACTGCTAGC[GCG=AAT]ATGACGCGCGGCTACTACATCAGCT
Deletion
>Sequence1
ACGGACTAGCAGTCGCAGACTG[CTAGCGCG=]ATGACGCGCGGCTACTACATCAGCT
Insertion
>Sequence1
ACGGACTAGCAGTCGCAGACTGCTAGCGCG[=ATCGTNNNGC]ATGACGCGCGGCTACTACATCAGCT

Example 1 - Multiple sequences into same site in vector backbone>Vector backbone ACGGACTAGCAGTCGCAGACTGCTACTGCCGATCGCGAGCGCTATCGACGCGATATCGCG GGCGAGTCATATCTCGTACTCTCTACGACTGCATCGAATCTTCACGAGGACTACTACGAC TACGATTATTACGTCCGCGCGGCATCGATCGATCGACTCGAAGCATCGTACTCGCATGCT AGCTGACTAGCTACACGTACATCTACTACTACGCTGCTCATCGCATACTTTATCATGCGC TACGCGCTACGTCGAGCTACGCTACG + >Gene1 ATGCGATGCTGGACCATTGACCAGCATAGCGTCGTATCGGGATGCTG >Gene2 ACGTGCTAGTACGACTAGCTGCTGACCATAGTCATGCATGCAGTGCA >Gene3 CGAGCTACGAACTATGCATCGCCGCTACTACGTATAGCTTATTCGGT +When choosing "circular output" (see below), the above input will result in 3 circular outputs:Vector backbone - Gene1 - (circular) Vector backbone - Gene2 - (circular) Vector backbone - Gene3 - (circular)Here the 5' end of the vector backbone will be fused to the 3' end of the genes, while the 3' end of the backbone will be fused to the 5' end of the gene.
Example 2 - Combinatorial assembly>Sequence1 ACGGACTAGCAGTCGCAGACTGCTAGCATGACGCGCGGCTACTACATCAGCT + >Sequence2 ATGCGATGCTGGACCATTGACCAGCATAGCGTCGTATCGGGATGCTG >Sequence3 ACGTGCTAGTACGACTAGCTGCTGACCATAGTCATGCATGCAGTGCA >Sequence4 CGAGCTACGAACTATGCATCGCCGCTACTACGTATAGCTTATTCGGT + >Sequence5 CGATGCATGACGACGACTACAGTCGTAGCATCGATCGATCGTCGATCGHere, primers will be generated for a total of 3 output sequences:Sequence1 - Sequence2 - Sequence5 Sequence1 - Sequence3 - Sequence5 Sequence1 - Sequence4 - Sequence5

Example - linear assembly
Input sequence
>Sequence1
ACGGACTAGCAGTCGCAGACTGCTAGCATGACGCGCGGCTACTACATCAGCT
>Sequence2
ATGCGATGCTGGACCATTGACCAGCATAGCGTCGTATCGGGATGCTG
Cassette: PacI/Nt.BbvCI
Output sequence
Sequence1 - Sequence2 (end are joined to a circular DNA molecule)
ACGGACTAGCAGTCGCAGACTGCTAGCATGACGCGCGGCTACTACATCAGCTATGCGA
TGCTGGACCATTGACCAGCATAGCGTCGTATCGGGATGCTG (Linear DNA)
Example - circular assembly
Input sequence
>Sequence1
ACGGACTAGCAGTCGCAGACTGCTAGCATGACGCGCGGCTACTACATCAGCT
>Sequence2
ATGCGATGCTGGACCATTGACCAGCATAGCGTCGTATCGGGATGCTG
Cassette: PacI/Nt.BbvCI
Output sequence
- Sequence1 - Sequence2 - (end are joined to a circular DNA molecule)
...ACGGACTAGCAGTCGCAGACTGCTAGCATGACGCGCGGCTACTACATCAGCTATGCGA
TGCTGGACCATTGACCAGCATAGCGTCGTATCGGGATGCTG... (Cicular DNA)
Example - USER cassette
Input sequence
>Sequence1
ACGGACTAGCAGTCGCAGACTGCTAGCATGACGCGCGGCTACTACATCAGCT
>Sequence2
ATGCGATGCTGGACCATTGACCAGCATAGCGTCGTATCGGGATGCTG
Cassette: PacI/Nt.BbvCI
Output sequence
PacI/Nt.BbvCI - Sequence1 - Sequence2 - PacI/Nt.BbvCI
PacI/Nt.BbvCIACGGACTAGCAGTCGCAGACTGCTAGCATGACGCGCGGCTACTACATCAGCTATGCGATGCTGGACCATT
GACCAGCATAGCGTCGTATCGGGATGCTGPacI/Nt.BbvCI
1: Name: PacI/Nt.BbvCI Forward strand: GCTGAGGGTTTAATTAAGACCTCAGC Reverse strand: CGACTCCCAAATTAATTCTGGAGTCG 2: Name: PacI/Nt.BbvCIXbaI2/Nt.BbvCI Forward strand: GCTGAGGGAAAGTCTAGAGGATCCTCTAGATGTCTCCTCAGC Reverse strand: CGACTCCCTTTCAGATCTCCTAGGAGATCTACAGAGGAGTCG 3: Name: PacI/Nt.BbvCIPmeI/Nb.BbvCI Forward strand: CCTCAGCCGTTTAAACAGCTGAGG Reverse strand: GGAGTCGGCAAATTTGTCGACTCC 4: Name: PacI/Nt.BbvCIAsiSI/Nb.BSMI Forward strand: GAATGCGTGCGATCGCGTGCATTC Reverse strand: CTTACGCACGCTAGCGCACGTAAG 5: Name: PacI/Nt.BbvCIAsiSI/Nb.BtsI Forward strand: GCAGTGAGAGCGATCGCAGACACTGC Reverse strand: CGTCACTCTCGCTAGCGTCTGTGACG
Forward: GCTGAGGGTTTAAT.TAAGACC.TCAGCwhere the first period indicates the position of the restriction site, and second period indicates the position of the nicking site.
Reverse: CGACT.CCCAAAT.TAATTCTGGAGTCGwhere first period indicates the position of the nicking site and second period indicates the position of the restriction site.
Back to topExample - User designed cassettes
Typing the following casette:
GCTGAGGGTTTAAT.TAAGACC.TCAGC
CGACT.CCCAAAT.TAATTCTGGAGTCG
Will result ing the following overhangs:
GCTGAGGGTTTAAT TCAGC
CGACT TAATTCTGGAGTCG
>ori TCTCCTGAGTAGGACAAATCCGCCGCCCTAGACCTAGGGCGTTCGGCTGCGGCGAGCGGT ATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAA GAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGC GTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAG GTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGT GCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGG AAGCGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCG CTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGG TAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCAC TGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTG GCCTAACTACGGCTACACTAGAAGGACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGT TACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGG TGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAGAAGATCC TTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTT GGTCATGACTAGTGCTTGGATTCTCACCAATAAAAAACGCCCGGCGGCAACCGAGCGTTC TGAACAAATCCAGATGGAGTTCTGAGGTCATTACTGGAT >selection marker AACAGGAGTCCAAGCGAGCTCTCGAACCCCAGAGTCCCGCTCAGAAGAACTCGTCAAGAA GGCGATAGAAGGCGATGCGCTGCGAATCGGGAGCGGCGATACCGTAAAGCACGAGGAAGC GGTCAGCCCATTCGCCGCCAAGCTCTTCAGCAATATCACGGGTAGCCAACGCTATGTCCT GATAGCGGTCCGCCACACCCAGCCGGCCACAGTCGATGAATCCAGAAAAGCGGCCATTTT CCACCATGATATTCGGCAAGCAGGCATCGCCATGGGTCACGACGAGATCCTCGCCGTCGG GCATGCGCGCCTTGAGCCTGGCGAACAGTTCGGCTGGCGCGAGCCCCTGATGCTCTTCGT CCAGATCATCCTGATCGACAAGACCGGCTTCCATCCGAGTACGTGCTCGCTCGATGCGAT GTTTCGCTTGGTGGTCGAATGGGCAGGTAGCCGGATCAAGCGTATGCAGCCGCCGCATTG CATCAGCCATGATGGATACTTTCTCGGCAGGAGCAAGGTGAGATGACAGGAGATCCTGCC CCGGCACTTCGCCCAATAGCAGCCAGTCCCTTCCCGCTTCAGTGACAACGTCGAGCACAG CTGCGCAAGGAACGCCCGTCGTGGCCAGCCACGATAGCCGCGCTGCCTCGTCCTGCAGTT CATTCAGGGCACCGGACAGGTCGGTCTTGACAAAAAGAACCGGGCGCCCCTGCGCTGACA GCCGGAACACGGCGGCATCAGAGCAGCCGATTGTCTGTTGTGCCCAGTCATAGCCGAATA GCCTCTCCACCCAAGCGGCCGGAGAACCTGCGTGCAATCCATCTTGTTCAATCATGCGAA ACGATCCTCATCCTGTCTCTTGATCAGATCTTGATCCCCTGCGCCATCAGATCCTTGGCG GCAAGAAAGCCATCCAGTTTACTTTGCAGGGCTTCCCAACCTTACCAGAGGGCGCCCCAG CTGGCAATTCCGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGC GTATCACGAGGCCCTTTCGTCTTCACCTCGAGTCCCTATCAGTGATAGAGATTGACATCC CTATCAGTGATAGAGATACTGAGCACATCAGCAGGACGCACTGACCGAATTCATTAAAGA GGAGAAAGGTACCG >CDS ATGCGTAAAGGAGAAGAACTTTTCACTGGAGTTGTCCCAATTCTTGTTGAATTAGATGGT GATGTTAATGGGCACAAATTTTCTGTCAGTGGAGAGGGTGAAGGTGATGCAACATACGGA AAACTTACCCTTAAATTTATTTGCACTACTGGAAAACTACCTGTTCCATGGCCAACACTT GTCACTACTTTCGGTTATGGTGTTCAATGCTTTGCGAGATACCCAGATCATATGAAACAG CATGACTTTTTCAAGAGTGCCATGCCCGAAGGTTATGTACAGGAAAGAACTATATTTTTC AAAGATGACGGGAACTACAAGACACGTGCTGAAGTCAAGTTTGAAGGTGATACCCTTGTT AATAGAATCGAGTTAAAAGGTATTGATTTTAAAGAAGATGGAAACATTCTTGGACACAAA TTGGAATACAACTATAACTCACACAATGTATACATCATGGCAGACAAACAAAAGAATGGA ATCAAAGTTAACTTCAAAATTAGACACAACATTGAAGATGGAAGCGTTCAACTAGCAGAC CATTATCAACAAAATACTCCAATTGGCGATGGCCCTGTCCTTTTACCAGACAACCATTAC CTGTCCACACAATCTGCCCTTTCGAAAGATCCCAACGAAAAGAGAGACCACATGGTCCTT CTTGAGTTTGTAACAGCTGCTGGGATTACACATGGCATGGATGAACTATACAAATAA |
|
-------------------------------------- AMUSER output generated: 30-12-2012 19:29:04 -------------------------------------- input parameters: ----------------- number of fragments: 3 salt concentration selected: 50 nM primer concentration selected: 0.5 uM circular assembly (no cassette) overview of the needed primers (5'-3'): --------------------------------------- forward primer for ori: ATAATCUCCTGAGTAGGACAAATCC reverse primer for ori: ACTCCTGTTAUCCAGTAATGACCTCAGAA forward primer for selection marker: ATAACAGGAGUCCAAGCGAGCTCTCGAAC reverse primer for selection marker: ACGCATCGGUACCTTTCTCCTCTTTAATGAATTC forward primer for CDS: ACCGATGCGUAAAGGAGAAGAACTTTTCA reverse primer for CDS: AGATTAUTTGTATAGTTCATCCATGC attention: one or more of the designed primers may have undesirable properties please see "primer details" section overview of your final construct after cloning (circular): ---------------------------------------------------------- ori (3' end) selection marker CDS ori (5' end) graphic overview of DNA fragments and primers: ---------------------------------------------- fusion region and related primers for joining of ori and selection marker: 5'-ATAACAGGAGU CCAAGCGAGCTCTCGAAC-3' 5'-[...]CTGAACAAATCCAGATGGAGTTCTGAGGTCATTACTGGATAACAGGAGTCCAAGCGAGCTCTCGAACCCCAGAGTCCCGC[...]-3' 3'-AAGACTCCAGTAATGACC UATTGTCCTCA-5' fusion region and related primers for joining of selection marker and CDS: 5'-ACCGATGCGU AAAGGAGAAGAACTTTTCA-3' 5'-[...]GACGCACTGACCGAATTCATTAAAGAGGAGAAAGGTACCGATGCGTAAAGGAGAAGAACTTTTCACTGGAGTTGTCCCAA[...]-3' 3'-CTTAAGTAATTTCTCCTCTTTCCA UGGCTACGCA-5' fusion region and related primers for joining of CDS and ori: 5'-ATAATCU CCTGAGTAGGACAAATCC-3' 5'-[...]TGCTGGGATTACACATGGCATGGATGAACTATACAAATAATCTCCTGAGTAGGACAAATCCGCCGCCCTAGACCTAGGGC[...]-3' 3'-CGTACCTACTTGATATGTT UATTAGA-5' primer details: --------------- For detailed descriptions of primer evaluation parameters, see instructions tab. primer details for ori forward primer: ATAATCUCCTGAGTAGGACAAATCC * Tm: 49.2C - in optimal range (55-72)? ...NO * GC content: 50.00% - in optimal range (40-60)? ...YES * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO reverse primer: ACTCCTGTTAUCCAGTAATGACCTCAGAA * Tm: 47.9C - in optimal range (55-72)? ...NO * GC content: 44.44% - in optimal range (40-60)? ...YES * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO Tm of primers within 2C of each other? ...YES primer details for selection marker forward primer: ATAACAGGAGUCCAAGCGAGCTCTCGAAC * Tm: 58.0C - in optimal range (55-72)? ...YES * GC content: 61.11% - in optimal range (40-60)? ...NO * GC clamp present at 3' end? ...NO * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO reverse primer: ACGCATCGGUACCTTTCTCCTCTTTAATGAATTC * Tm: 54.1C - in optimal range (55-72)? ...NO * GC content: 33.33% - in optimal range (40-60)? ...NO * GC clamp present at 3' end? ...NO * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO Tm of primers within 2C of each other? ...NO primer details for CDS forward primer: ACCGATGCGUAAAGGAGAAGAACTTTTCA * Tm: 48.0C - in optimal range (55-72)? ...NO * GC content: 31.58% - in optimal range (40-60)? ...NO * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...YES reverse primer: AGATTAUTTGTATAGTTCATCCATGC * Tm: 47.6C - in optimal range (55-72)? ...NO * GC content: 36.84% - in optimal range (40-60)? ...NO * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...YES Tm of primers within 2C of each other? ...YES details of final construct after cloning (circular, length: 2810 bases): ------------------------------------------------------------------------ ori selection marker CDS 1 TCTCCTGAGTAGGACAAATCCGCCGCCCTAGACCTAGGGCGTTCGGCTGCGGCGAGCGGT 61 ATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAA 121 GAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGC 181 GTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAG 241 GTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGT 301 GCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGG 361 AAGCGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCG 421 CTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGG 481 TAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCAC 541 TGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTG 601 GCCTAACTACGGCTACACTAGAAGGACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGT 661 TACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGG 721 TGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAGAAGATCC 781 TTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTT 841 GGTCATGACTAGTGCTTGGATTCTCACCAATAAAAAACGCCCGGCGGCAACCGAGCGTTC 901 TGAACAAATCCAGATGGAGTTCTGAGGTCATTACTGGATAACAGGAGTCCAAGCGAGCTC 961 TCGAACCCCAGAGTCCCGCTCAGAAGAACTCGTCAAGAAGGCGATAGAAGGCGATGCGCT 1021 GCGAATCGGGAGCGGCGATACCGTAAAGCACGAGGAAGCGGTCAGCCCATTCGCCGCCAA 1081 GCTCTTCAGCAATATCACGGGTAGCCAACGCTATGTCCTGATAGCGGTCCGCCACACCCA 1141 GCCGGCCACAGTCGATGAATCCAGAAAAGCGGCCATTTTCCACCATGATATTCGGCAAGC 1201 AGGCATCGCCATGGGTCACGACGAGATCCTCGCCGTCGGGCATGCGCGCCTTGAGCCTGG 1261 CGAACAGTTCGGCTGGCGCGAGCCCCTGATGCTCTTCGTCCAGATCATCCTGATCGACAA 1321 GACCGGCTTCCATCCGAGTACGTGCTCGCTCGATGCGATGTTTCGCTTGGTGGTCGAATG 1381 GGCAGGTAGCCGGATCAAGCGTATGCAGCCGCCGCATTGCATCAGCCATGATGGATACTT 1441 TCTCGGCAGGAGCAAGGTGAGATGACAGGAGATCCTGCCCCGGCACTTCGCCCAATAGCA 1501 GCCAGTCCCTTCCCGCTTCAGTGACAACGTCGAGCACAGCTGCGCAAGGAACGCCCGTCG 1561 TGGCCAGCCACGATAGCCGCGCTGCCTCGTCCTGCAGTTCATTCAGGGCACCGGACAGGT 1621 CGGTCTTGACAAAAAGAACCGGGCGCCCCTGCGCTGACAGCCGGAACACGGCGGCATCAG 1681 AGCAGCCGATTGTCTGTTGTGCCCAGTCATAGCCGAATAGCCTCTCCACCCAAGCGGCCG 1741 GAGAACCTGCGTGCAATCCATCTTGTTCAATCATGCGAAACGATCCTCATCCTGTCTCTT 1801 GATCAGATCTTGATCCCCTGCGCCATCAGATCCTTGGCGGCAAGAAAGCCATCCAGTTTA 1861 CTTTGCAGGGCTTCCCAACCTTACCAGAGGGCGCCCCAGCTGGCAATTCCGACGTCTAAG 1921 AAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTC 1981 TTCACCTCGAGTCCCTATCAGTGATAGAGATTGACATCCCTATCAGTGATAGAGATACTG 2041 AGCACATCAGCAGGACGCACTGACCGAATTCATTAAAGAGGAGAAAGGTACCGATGCGTA 2101 AAGGAGAAGAACTTTTCACTGGAGTTGTCCCAATTCTTGTTGAATTAGATGGTGATGTTA 2161 ATGGGCACAAATTTTCTGTCAGTGGAGAGGGTGAAGGTGATGCAACATACGGAAAACTTA 2221 CCCTTAAATTTATTTGCACTACTGGAAAACTACCTGTTCCATGGCCAACACTTGTCACTA 2281 CTTTCGGTTATGGTGTTCAATGCTTTGCGAGATACCCAGATCATATGAAACAGCATGACT 2341 TTTTCAAGAGTGCCATGCCCGAAGGTTATGTACAGGAAAGAACTATATTTTTCAAAGATG 2401 ACGGGAACTACAAGACACGTGCTGAAGTCAAGTTTGAAGGTGATACCCTTGTTAATAGAA 2461 TCGAGTTAAAAGGTATTGATTTTAAAGAAGATGGAAACATTCTTGGACACAAATTGGAAT 2521 ACAACTATAACTCACACAATGTATACATCATGGCAGACAAACAAAAGAATGGAATCAAAG 2581 TTAACTTCAAAATTAGACACAACATTGAAGATGGAAGCGTTCAACTAGCAGACCATTATC 2641 AACAAAATACTCCAATTGGCGATGGCCCTGTCCTTTTACCAGACAACCATTACCTGTCCA 2701 CACAATCTGCCCTTTCGAAAGATCCCAACGAAAAGAGAGACCACATGGTCCTTCTTGAGT 2761 TTGTAACAGCTGCTGGGATTACACATGGCATGGATGAACTATACAAATAA primers in FASTA format (5'-3') ------------------------------- >FW_ori_[Tm:49.6] ATAATCUCCTGAGTAGGACAAATCC >RV_ori_[Tm:47.9] ACTCCTGTTAUCCAGTAATGACCTCAGAA >FW_selection marker_[Tm:58.0] ATAACAGGAGUCCAAGCGAGCTCTCGAAC >RV_selection marker_[Tm:54.1] ACGCATCGGUACCTTTCTCCTCTTTAATGAATTC >FW_CDS_[Tm:48.0] ACCGATGCGUAAAGGAGAAGAACTTTTCA >RV_CDS_[Tm:47.6] AGATTAUTTGTATAGTTCATCCATGC |
|
>vectorbackbone_with_GFP CTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAG TTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGGACAGTATTTGGTATCTGCGCT CTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACC ACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGA TCTCAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCA CGTTAAGGGATTTTGGTCATGACTAGTGCTTGGATTCTCACCAATAAAAAACGCCCGGCG GCAACCGAGCGTTCTGAACAAATCCAGATGGAGTTCTGAGGTCATTACTGGATAACAGGA GTCCAAGCGAGCTCTCGAACCCCAGAGTCCCGCTCAGAAGAACTCGTCAAGAAGGCGATA GAAGGCGATGCGCTGCGAATCGGGAGCGGCGATACCGTAAAGCACGAGGAAGCGGTCAGC CCATTCGCCGCCAAGCTCTTCAGCAATATCACGGGTAGCCAACGCTATGTCCTGATAGCG GTCCGCCACACCCAGCCGGCCACAGTCGATGAATCCAGAAAAGCGGCCATTTTCCACCAT GATATTCGGCAAGCAGGCATCGCCATGGGTCACGACGAGATCCTCGCCGTCGGGCATGCG CGCCTTGAGCCTGGCGAACAGTTCGGCTGGCGCGAGCCCCTGATGCTCTTCGTCCAGATC ATCCTGATCGACAAGACCGGCTTCCATCCGAGTACGTGCTCGCTCGATGCGATGTTTCGC TTGGTGGTCGAATGGGCAGGTAGCCGGATCAAGCGTATGCAGCCGCCGCATTGCATCAGC CATGATGGATACTTTCTCGGCAGGAGCAAGGTGAGATGACAGGAGATCCTGCCCCGGCAC TTCGCCCAATAGCAGCCAGTCCCTTCCCGCTTCAGTGACAACGTCGAGCACAGCTGCGCA AGGAACGCCCGTCGTGGCCAGCCACGATAGCCGCGCTGCCTCGTCCTGCAGTTCATTCAG GGCACCGGACAGGTCGGTCTTGACAAAAAGAACCGGGCGCCCCTGCGCTGACAGCCGGAA CACGGCGGCATCAGAGCAGCCGATTGTCTGTTGTGCCCAGTCATAGCCGAATAGCCTCTC CACCCAAGCGGCCGGAGAACCTGCGTGCAATCCATCTTGTTCAATCATGCGAAACGATCC TCATCCTGTCTCTTGATCAGATCTTGATCCCCTGCGCCATCAGATCCTTGGCGGCAAGAA AGCCATCCAGTTTACTTTGCAGGGCTTCCCAACCTTACCAGAGGGCGCCCCAGCTGGCAA TTCCGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCAC GAGGCCCTTTCGTCTTCACCTCGAGTCCCTATCAGTGATAGAGATTGACATCCCTATCAG TGATAGAGATACTGAGCACATCAGCAGGACGCACTGACCGAATTCATTAAAGAGGAGAAA GGTACCGATGCGTAAAGGAGAAGAACTTTTCACTGGAGTTGTCCCAATTCTTGTTGAATT AGATGGTGATGTTAATGGGCACAAATTTTCTGTCAGTGGAGAGGGTGAAGGTGATGCAAC ATACGGAAAACTTACCCTTAAATTTATTTGCACTACTGGAAAACTACCTGTTCCATGGCC AACACTTGTCACTACTTTCGGTTATGGTGTTCAATGCTTTGCGAGATACCCAGATCATAT GAAACAGCATGACTTTTTCAAGAGTGCCATGCCCGAAGGTTATGTACAGGAAAGAACTAT ATTTTTCAAAGATGACGGGAACTACAAGACACGTGCTGAAGTCAAGTTTGAAGGTGATAC CCTTGTTAATAGAATCGAGTTAAAAGGTATTGATTTTAAAGAAGATGGAAACATTCTTGG ACACAAATTGGAATACAACTATAACTCACACAATGTATACATCATGGCAGACAAACAAAA GAATGGAATCAAAGTTAACTTCAAAATTAGACACAACATTGAAGATGGAAGCGTTCAACT AGCAGACCATTATCAACAAAATACTCCAATTGGCGATGGCCCTGTCCTTTTA[CCA=AAA ]GACAACCATTACCTGTCCACACAATCTGCCCTTTCGAAAGATCCCAACGAAAAGAGAGA CCACATGGTCCTTCTTGAGTTTGTAACAGCTGCTGGGATTACACATGGCATGGATGAACT ATACAAA[=CATCATCACCATCACCAC]TAATCTCCTGAGTAGGACAAATCCGCCGCCCT AGACCTAGGGCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGT TATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGG CCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACG AGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGAT ACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTA CCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCACGCT GTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCC CCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAA GACACGACTTATCGCCA |
|
-------------------------------------- AMUSER output generated: 30-12-2012 20:08:35 -------------------------------------- input parameters: ----------------- number of fragments: 1 number of inserts: 1 salt concentration selected: 50 nM primer concentration selected: 0.5 uM circular assembly (no cassette) overview of the needed primers (5'-3'): --------------------------------------- forward primer for vectorbackbone_with_GFP: ATCGCCACUGGCAGCAGCCACTGGTAA reverse primer for vectorbackbone_with_GFP: ATGGTTGTCTTUTAAAAGGACAGGGCCATCGCCAAT forward primer for vectorbackbone_with_GFP: AAAGACAACCAUTACCTGTCCACACAATCT reverse primer for vectorbackbone_with_GFP: ATGGTGAUGATGTTTGTATAGTTCATCCATGC forward primer for vectorbackbone_with_GFP: ATCACCAUCACCACTAATCTCCTGAGTAGGACAAAT reverse primer for vectorbackbone_with_GFP: AGTGGCGAUAAGTCGTGTCTTACCGGG attention: one or more of the designed primers may have undesirable properties please see "primer details" section overview of your final construct after cloning (circular): ---------------------------------------------------------- vectorbackbone_with_GFP (3' end) vectorbackbone_with_GFP insert1 vectorbackbone_with_GFP vectorbackbone_with_GFP (5' end) graphic overview of DNA fragments and primers: ---------------------------------------------- fusion region and related primers for joining of vectorbackbone_with_GFP and vectorbackbone_with_GFP: 5'-AAAGACAACCAU TACCTGTCCACACAATCT-3' 5'-[...]CAACAAAATACTCCAATTGGCGATGGCCCTGTCCTTTTAAAAGACAACCATTACCTGTCCACACAATCTGCCCTTTCGAA[...]-3' 3'-TAACCGCTACCGGGACAGGAAAAT UTTCTGTTGGTA-5' fusion region and related primers for joining of vectorbackbone_with_GFP and vectorbackbone_with_GFP: 5'-ATCACCAU CACCACTAATCTCCTGAGTAGGACAAAT-3' 5'-[...]AGCTGCTGGGATTACACATGGCATGGATGAACTATACAAACATCATCACCATCACCACTAATCTCCTGAGTAGGACAAATCCGCCGCCCTAGACCTAG[...]-3' 3'-CGTACCTACTTGATATGTTTGTAG UAGTGGTA-5' fusion region and related primers for joining of vectorbackbone_with_GFP and vectorbackbone_with_GFP: 5'-ATCGCCACU GGCAGCAGCCACTGGTAA-3' 5'-[...]TCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGT[...]-3' 3'-GGGCCATTCTGTGCTGAA UAGCGGTGA-5' primer details: --------------- For detailed descriptions of primer evaluation parameters, see instructions tab. primer details for vectorbackbone_with_GFP forward primer: ATCGCCACUGGCAGCAGCCACTGGTAA * Tm: 56.9C - in optimal range (55-72)? ...YES * GC content: 60.00% - in optimal range (40-60)? ...YES * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO reverse primer: ATGGTTGTCTTUTAAAAGGACAGGGCCATCGCCAAT * Tm: 60.7C - in optimal range (55-72)? ...YES * GC content: 50.00% - in optimal range (40-60)? ...YES * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...YES * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO Tm of primers within 2C of each other? ...YES primer details for vectorbackbone_with_GFP forward primer: AAAGACAACCAUTACCTGTCCACACAATCT * Tm: 49.0C - in optimal range (55-72)? ...NO * GC content: 44.44% - in optimal range (40-60)? ...YES * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO reverse primer: ATGGTGAUGATGTTTGTATAGTTCATCCATGC * Tm: 48.8C - in optimal range (55-72)? ...NO * GC content: 35.00% - in optimal range (40-60)? ...NO * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO Tm of primers within 2C of each other? ...YES primer details for vectorbackbone_with_GFP forward primer: ATCACCAUCACCACTAATCTCCTGAGTAGGACAAAT * Tm: 52.8C - in optimal range (55-72)? ...NO * GC content: 36.36% - in optimal range (40-60)? ...NO * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO reverse primer: AGTGGCGAUAAGTCGTGTCTTACCGGG * Tm: 54.5C - in optimal range (55-72)? ...NO * GC content: 55.56% - in optimal range (40-60)? ...YES * GC clamp present at 3' end? ...YES * more than 3 G/C out of last 5 bases at 3' end? ...NO * risk of primer dimer formation in primer pair? ...NO * risk of intra-primer homology (secondary structures)? ...NO * presence of polyN stretches? ...NO Tm of primers within 2C of each other? ...YES details of final construct after cloning (circular, length: 2828 bases): ------------------------------------------------------------------------ vectorbackbone_with_GFP vectorbackbone_with_GFP insert1 vectorbackbone_with_GFP 1 CTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAG 61 TTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGGACAGTATTTGGTATCTGCGCT 121 CTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACC 181 ACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGA 241 TCTCAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCA 301 CGTTAAGGGATTTTGGTCATGACTAGTGCTTGGATTCTCACCAATAAAAAACGCCCGGCG 361 GCAACCGAGCGTTCTGAACAAATCCAGATGGAGTTCTGAGGTCATTACTGGATAACAGGA 421 GTCCAAGCGAGCTCTCGAACCCCAGAGTCCCGCTCAGAAGAACTCGTCAAGAAGGCGATA 481 GAAGGCGATGCGCTGCGAATCGGGAGCGGCGATACCGTAAAGCACGAGGAAGCGGTCAGC 541 CCATTCGCCGCCAAGCTCTTCAGCAATATCACGGGTAGCCAACGCTATGTCCTGATAGCG 601 GTCCGCCACACCCAGCCGGCCACAGTCGATGAATCCAGAAAAGCGGCCATTTTCCACCAT 661 GATATTCGGCAAGCAGGCATCGCCATGGGTCACGACGAGATCCTCGCCGTCGGGCATGCG 721 CGCCTTGAGCCTGGCGAACAGTTCGGCTGGCGCGAGCCCCTGATGCTCTTCGTCCAGATC 781 ATCCTGATCGACAAGACCGGCTTCCATCCGAGTACGTGCTCGCTCGATGCGATGTTTCGC 841 TTGGTGGTCGAATGGGCAGGTAGCCGGATCAAGCGTATGCAGCCGCCGCATTGCATCAGC 901 CATGATGGATACTTTCTCGGCAGGAGCAAGGTGAGATGACAGGAGATCCTGCCCCGGCAC 961 TTCGCCCAATAGCAGCCAGTCCCTTCCCGCTTCAGTGACAACGTCGAGCACAGCTGCGCA 1021 AGGAACGCCCGTCGTGGCCAGCCACGATAGCCGCGCTGCCTCGTCCTGCAGTTCATTCAG 1081 GGCACCGGACAGGTCGGTCTTGACAAAAAGAACCGGGCGCCCCTGCGCTGACAGCCGGAA 1141 CACGGCGGCATCAGAGCAGCCGATTGTCTGTTGTGCCCAGTCATAGCCGAATAGCCTCTC 1201 CACCCAAGCGGCCGGAGAACCTGCGTGCAATCCATCTTGTTCAATCATGCGAAACGATCC 1261 TCATCCTGTCTCTTGATCAGATCTTGATCCCCTGCGCCATCAGATCCTTGGCGGCAAGAA 1321 AGCCATCCAGTTTACTTTGCAGGGCTTCCCAACCTTACCAGAGGGCGCCCCAGCTGGCAA 1381 TTCCGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCAC 1441 GAGGCCCTTTCGTCTTCACCTCGAGTCCCTATCAGTGATAGAGATTGACATCCCTATCAG 1501 TGATAGAGATACTGAGCACATCAGCAGGACGCACTGACCGAATTCATTAAAGAGGAGAAA 1561 GGTACCGATGCGTAAAGGAGAAGAACTTTTCACTGGAGTTGTCCCAATTCTTGTTGAATT 1621 AGATGGTGATGTTAATGGGCACAAATTTTCTGTCAGTGGAGAGGGTGAAGGTGATGCAAC 1681 ATACGGAAAACTTACCCTTAAATTTATTTGCACTACTGGAAAACTACCTGTTCCATGGCC 1741 AACACTTGTCACTACTTTCGGTTATGGTGTTCAATGCTTTGCGAGATACCCAGATCATAT 1801 GAAACAGCATGACTTTTTCAAGAGTGCCATGCCCGAAGGTTATGTACAGGAAAGAACTAT 1861 ATTTTTCAAAGATGACGGGAACTACAAGACACGTGCTGAAGTCAAGTTTGAAGGTGATAC 1921 CCTTGTTAATAGAATCGAGTTAAAAGGTATTGATTTTAAAGAAGATGGAAACATTCTTGG 1981 ACACAAATTGGAATACAACTATAACTCACACAATGTATACATCATGGCAGACAAACAAAA 2041 GAATGGAATCAAAGTTAACTTCAAAATTAGACACAACATTGAAGATGGAAGCGTTCAACT 2101 AGCAGACCATTATCAACAAAATACTCCAATTGGCGATGGCCCTGTCCTTTTAAAAGACAA 2161 CCATTACCTGTCCACACAATCTGCCCTTTCGAAAGATCCCAACGAAAAGAGAGACCACAT 2221 GGTCCTTCTTGAGTTTGTAACAGCTGCTGGGATTACACATGGCATGGATGAACTATACAA 2281 ACATCATCACCATCACCACTAATCTCCTGAGTAGGACAAATCCGCCGCCCTAGACCTAGG 2341 GCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAG 2401 AATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACC 2461 GTAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACA 2521 AAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGT 2581 TTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACC 2641 TGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATC 2701 TCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGC 2761 CCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACT 2821 TATCGCCA primers in FASTA format (5'-3') ------------------------------- >FW_vectorbackbone_with_GFP_[Tm:59.9] ATCGCCACUGGCAGCAGCCACTGGTAA >RV_vectorbackbone_with_GFP_[Tm:60.7] ATGGTTGTCTTUTAAAAGGACAGGGCCATCGCCAAT >FW_vectorbackbone_with_GFP_[Tm:49.0] AAAGACAACCAUTACCTGTCCACACAATCT >RV_vectorbackbone_with_GFP_[Tm:48.8] ATGGTGAUGATGTTTGTATAGTTCATCCATGC >FW_vectorbackbone_with_GFP_[Tm:52.8] ATCACCAUCACCACTAATCTCCTGAGTAGGACAAAT >RV_vectorbackbone_with_GFP_[Tm:54.5] AGTGGCGAUAAGTCGTGTCTTACCGGG |
>front_vector_backbone_minus_ori AACAGGAGTCCAAGCGAGCTCTCGAACCCCAGAGTCCCGCTCAGAAGAACTCGTCAAGAA GGCGATAGAAGGCGATGCGCTGCGAATCGGGAGCGGCGATACCGTAAAGCACGAGGAAGC GGTCAGCCCATTCGCCGCCAAGCTCTTCAGCAATATCACGGGTAGCCAACGCTATGTCCT GATAGCGGTCCGCCACACCCAGCCGGCCACAGTCGATGAATCCAGAAAAGCGGCCATTTT CCACCATGATATTCGGCAAGCAGGCATCGCCATGGGTCACGACGAGATCCTCGCCGTCGG GCATGCGCGCCTTGAGCCTGGCGAACAGTTCGGCTGGCGCGAGCCCCTGATGCTCTTCGT CCAGATCATCCTGATCGACAAGACCGGCTTCCATCCGAGTACGTGCTCGCTCGATGCGAT GTTTCGCTTGGTGGTCGAATGGGCAGGTAGCCGGATCAAGCGTATGCAGCCGCCGCATTG CATCAGCCATGATGGATACTTTCTCGGCAGGAGCAAGGTGAGATGACAGGAGATCCTGCC CCGGCACTTCGCCCAATAGCAGCCAGTCCCTTCCCGCTTCAGTGACAACGTCGAGCACAG CTGCGCAAGGAACGCCCGTCGTGGCCAGCCACGATAGCCGCGCTGCCTCGTCCTGCAGTT CATTCAGGGCACCGGACAGGTCGGTCTTGACAAAAAGAACCGGGCGCCCCTGCGCTGACA GCCGGAACACGGCGGCATCAGAGCAGCCGATTGTCTGTTGTGCCCAGTCATAGCCGAATA GCCTCTCCACCCAAGCGGCCGGAGAACCTGCGTGCAATCCATCTTGTTCAATCATGCGAA ACGATCCTCATCCTGTCTCTTGATCAGATCTTGATCCCCTGCGCCATCAGATCCTTGGCG GCAAGAAAGCCATCCAGTTTACTTTGCAGGGCTTCCCAACCTTACCAGAGGGCGCCCCAG CTGGCAATTCCGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGC GTATCACGAGGCCCTTTCGTCTTCACCTCGAGTCCCTATCAGTGATAGAGATTGACATCC CTATCAGTGATAGAGATACTGAGCACATCAGCAGGACGCACTGACCGAATTCATTAAAGA GGAGAAAGGTACCGATGCGTAAAGGAGAAGAACTTTTCACTGGAGTTGTCCCAATTCTTG TTGAATTAGATGGTGATGTTAATGGGCACAAATTTTCTGTCAGTGGAGAGGGTGAAGGTG ATGCAACATACGGAAAACTTACCCTTAAATTTATTTGCACTACTGGAAAACTACCTGTTC CATGGCCAACACTTGTCACTACTTTCGGTTATGGTGTTCAATGCTTTGCGAGATACCCAG ATCATATGAAACAGCATGACTTTTTCAAGAGTGCCATGCCCGAAGGTTATGTACAGGAAA GAACTATATTTTTCAAAGATGACGGGAACTACAAGACACGTGCTGAAGTCAAGTTTGAAG GTGATACCCTTGTTAATAGAATCGAGTTAAAAGGTATTGATTTTAAAGAAGATGGAAACA TTCTTGGACACAAATTGGAATACAACTATAACTCACACAATGTATACATCATGGCAGACA AACAAAAGAATGGAATCAAAGTTAACTTCAAAATTAGACACAACATTGAAGATGGAAGCG TTCAACTAGCAGACCATTATCAACAAAATACTCCAATTGGCGATGGCCCTGTCCTTTTAC CAGACAACCATTACCTGTCCACACAATCTGCCCTTTCGAAAGATCCCAACGAAAAGAGAG ACCACATGGTCCTTCTTGAGTTTGTAACAGCTGCTGGGATTACACATGGCATGGATGAAC TATACAAATAA + >ori1 TCTCCTGAGTAGGACAAATCCGCCGCCCTAGACCTAGGGCGTTCGGCTGCGGCGAGCGGT ATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAA GAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGC GTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAG GTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGT GCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGG AAGCGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCG CTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGG TAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCAC TGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTG GCCTAACTACGGCTACACTAGAAGGACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGT TACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGG TGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAGAAGATCC TTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTT GGTCATGACTAGTGCTTGGATTCTCACCAATAAAAAACGCCCGGCGGCAACCGAGCGTTC TGAACAAATCCAGATGGAGTTCTGAGGTCATTACTGGAT >ori2 GCGCTAGCGGAGTGTATACTGGCTTACTATGTTGGCACTGATGAGGGTGTCAGTGAAGTG CTTCATGTGGCAGGAGAAAAAAGGCTGCACCGGTGCGTCAGCAGAATATGTGATACAGGA TATATTCCGCTTCCTCGCTCACTGACTCGCTACGCTCGGTCGTTCGACTGCGGCGAGCGG AAATGGCTTACGAACGGGGCGGAGATTTCCTGGAAGATGCCAGGAAGATACTTAACAGGG AAGTGAGAGGGCCGCGGCAAAGCCGTTTTTCCATAGGCTCCGCCCCCCTGACAAGCATCA CGAAATCTGACGCTCAAATCAGTGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGC GTTTCCCCCTGGCGGCTCCCTCGTGCGCTCTCCTGTTCCTGCCTTTCGGTTTACCGGTGT CATTCCGCTGTTATGGCCGCGTTTGTCTCATTCCACGCCTGACACTCAGTTCCGGGTAGG CAGTTCGCTCCAAGCTGGACTGTATGCACGAACCCCCCGTTCAGTCCGACCGCTGCGCCT TATCCGGTAACTATCGTCTTGAGTCCAACCCGGAAAGACATGCAAAAGCACCACTGGCAG CAGCCACTGGTAATTGATTTAGAGGAGTTAGTCTTGAAGTCATGCGCCGGTTAAGGCTAA ACTGAAAGGACAAGTTTTGGTGACTGCGCTCCTCCAAGCCAGTTACCTCGGTTCAAAGAG TTGGTAGCTCAGAGAACCTTCGAAAAACCGCCCTGCAAGGCGGTTTTTTCGTTTTCAGAG CAAGAGATTACGCGCAGACCAAAACGATCTCAAGAAGATCATCTTATTAATCAGATAAAA TATTTCTAGATTTCAGTGCAATTTATCTCTTCAAATGTAGCACCTGAAGTCAGCCCCATA CGATATAAGTTGT >ori3 AGATCAAAGGATCTTCTTGAGATCCTTTTTTTCTGCGCGTAATCTGCTGCTTGCAAACAA AAAAACCACCGCTACCAGCGGTGGTTTGTTTGCCGGATCAAGAGCTACCAACTCTTTTTC CGAAGGTAACTGGCTTCAGCAGAGCGCAGATACCAAATACTGTCCTTCTAGTGTAGCCGT AGTTAGGCCACCACTTCAAGAACTCTGTAGCACCGCCTACATACCTCGCTCTGCTAATCC TGTTACCAGTGGCTGCTGCCAGTGGCGATAAGTCGTGTCTTACCGGGTTGGACTCAAGAC GATAGTTACCGGATAAGGCGCAGCGGTCGGGCTGAACGGGGGGTTCGTGCACACAGCCCA GCTTGGAGCGAACGACCTACACCGAACTGAGATACCTACAGCGTGAGCTATGAGAAAGCG CCACGCTTCCCGAAGGGAGAAAGGCGGACAGGTATCCGGTAAGCGGCAGGGTCGGAACAG GAGAGCGCACGAGGGAGCTTCCAGGGGGAAACGCCTGGTATCTTTATAGTCCTGTCGGGT TTCGCCACCTCTGACTTGAGCGTCGATTTTTGTGATGCTCGTCAGGGGGGCGGAGCCTAT GGAAAAACGCCAGCAACGCG + >back_vector_backbone_minus_ori GCTTCCCAACCTTACCAGAGGGCGCCCCAG CTGGCAATTCCGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGC GTATCACGAGGCCCTTTCGTCTTCACCTCGAGTCCCTATCAGTGATAGAGATTGACATCC CTATCAGTGATAGAGATACTGAGCACATCAGCAGGACGCACTGACCGAATTCATTAAAGA GGAGAAAGGTACCGATGCGTAAAGGAGAAGAACTTTTCACTGGAGTTGTCCCAATTCTTG TTGAATTAGATGGTGATGTTAATGGGCACAAATTTTCTGTCAGTGGAGAGGGTGAAGGTG ATGCAACATACGGAAAACTTACCCTTAAATTTATTTGCACTACTGGAAAACTACCTGTTC CATGGCCAACACTTGTCACTACTTTCGGTTATGGTGTTCAATGCTTTGCGAGATACCCAG ATCATATGAAACAGCATGACTTTTTCAAGAGTGCCATGCCCGAAGGTTATGTACAGGAAA GAACTATATTTTTCAAAGATGACGGGAACTACAAGACACGTGCTGAAGTCAAGTTTGAAG GTGATACCCTTGTTAATAGAATCGAGTTAAAAGGTATTGATTTTAAAGAAGATGGAAACA TTCTTGGACACAAATTGGAATACAACTATAACTCACACAATGTATACATCATGGCAGACA AACAAAAGAATGGAATCAAAGTTAACTTCAAAATTAGACACAACATTGAAGATGGAAGCG TTCAACTAGCAGACCATTATCAACAAAATACTCCAATTGGCGATGGCCCTGTCCTTTTAC CAGACAACCATTACCTGTCCACACAATCTGCCCTTTCGAAAGATCCCAACGAAAAGAGAG ACCACATGGTCCTTCTTGAGTTTGTAACAGCTGCTGGGATTACACATGGCATGGATGAAC TATACAAATAAAACAGGAGTCCAAGCGAGCTCTCGAACCCCAGAGTCCCGCTCAGAAGAA GGCGATAGAAGGCGATGCGCTGCGAATCGGGAGCGGCGATACCGTAAAGCACGAGGAAGC GGTCAGCCCATTCGCCGCCAAGCTCTTCAGCAATATCACGGGTAGCCAACGCTATGTCCT GATAGCGGTCCGCCACACCCAGCCGGCCACAGTCGATGAATCCAGAAAAGCGGCCATTTT CCACCATGATATTCGGCAAGCAGGCATCGCCATGGGTCACGACGAGATCCTCGCCGTCGG GCATGCGCGCCTTGAGCCTGGCGAACAGTTCGGCTGGCGCGAGCCCCTGATGCTCTTCGT CCAGATCATCCTGATCGACAAGACCGGCTTCCATCCGAGTACGTGCTCGCTCGATGCGAT GTTTCGCTTGGTGGTCGAATGGGCAGGTAGCCGGATCAAGCGTATGCAGCCGCCGCATTG CATCAGCCATGATGGATACTTTCTCGGCAGGAGCAAGGTGAGATGACAGGAGATCCTGCC CCGGCACTTCGCCCAATAGCAGCCAGTCCCTTCCCGCTTCAGTGACAACGTCGAGCACAG CTGCGCAAGGAACGCCCGTCGTGGCCAGCCACGATAGCCGCGCTGCCTCGTCCTGCAGTT CATTCAGGGCACCGGACAGGTCGGTCTTGACAAAAAGAACCGGGCGCCCCTGCGCTGACA GCCGGAACACGGCGGCATCAGAGCAGCCGATTGTCTGTTGTGCCCAGTCATAGCCGAATA GCCTCTCCACCCAAGCGGCCGGAGAACCTGCGTGCAATCCATCTTGTTCAATCATGCGAA ACGATCCTCATCCTGTCTCTTGATCAGATCTTGATCCCCTGCGCCATCAGATCCTTGGCG GCAAGAAAGCCATCCAGTTTACTTTGCAGGCTCGTCAAGAA |
|
-------------------------------------- AMUSER output generated: 30-12-2012 21:12:45 -------------------------------------- input parameters: ----------------- number of batches: 3 circular assembly (no cassette) primers in FASTA format (5'-3') ------------------------------- batch 1 >FW_front_vector_backbone_minus_ori_[Tm:51.3] AAACAGGAGUCCAAGCGAGCTCTCGAAC >RV_front_vector_backbone_minus_ori_[Tm:50.1] AGATTAUTTGTATAGTTCATCCATGCCATG >FW_ori1_[Tm:45.4] ATAATCUCCTGAGTAGGACAAATCC >RV_ori1_[Tm:45.5] AGCATCCAGUAATGACCTCAGAACTCCA >FW_back_vector_backbone_minus_ori_[Tm:50.8] ACTGGATGCUTCCCAACCTTACCAGAGGG >RV_back_vector_backbone_minus_ori_[Tm:50.9] ACTCCTGTTUTCTTGACGAGCCTGCAAA batch 2 >FW_front_vector_backbone_minus_ori_[Tm:51.3] AAACAGGAGUCCAAGCGAGCTCTCGAAC >RV_front_vector_backbone_minus_ori_[Tm:50.1] AGCGCTTAUTTGTATAGTTCATCCATGCCATG >FW_ori2_[Tm:50.4] ATAAGCGCUAGCGGAGTGTATACTGGC >RV_ori2_[Tm:50.0] AGCACAACUTATATCGTATGGGGCTGACT >FW_back_vector_backbone_minus_ori_[Tm:50.8] AGTTGTGCUTCCCAACCTTACCAGAGGG >RV_back_vector_backbone_minus_ori_[Tm:50.9] ACTCCTGTTUTCTTGACGAGCCTGCAAA batch 3 >FW_front_vector_backbone_minus_ori_[Tm:51.3] AAACAGGAGUCCAAGCGAGCTCTCGAAC >RV_front_vector_backbone_minus_ori_[Tm:50.1] ATCTTTAUTTGTATAGTTCATCCATGCCATG >FW_ori3_[Tm:47.0] ATAAAGAUCAAAGGATCTTCTTGAGATC >RV_ori3_[Tm:48.3] AAGCCGCGUTGCTGGCGTTTTTCCATA >FW_back_vector_backbone_minus_ori_[Tm:49.3] ACGCGGCTUCCCAACCTTACCAGAGGG >RV_back_vector_backbone_minus_ori_[Tm:50.9] ACTCCTGTTUTCTTGACGAGCCTGCAAA |
Tm of primers: AMUSER ranks the Tm of primers on whether it is in the range 55-72 C. Primers for which the Tm falls in this region are marked with a green "Yes", primers for which the Tm falls outside this region are marked with a red "No".
GC content: The GC content (number of dGTP plus dCTP as a percentage of the total number of nucleic acid residues) should ideally be between 40-60 %, to keep annealing temperature within a favorable range. Primers for which the GC content falls in this region are marked with a green "Yes", primers for which the GC content falls outside this region are marked with a red "No".
Presence of 3' end GC clamp: Due to the stronger annealing of guanine and cytosine with complementary bases, specific binding is promoted by the presence of at least one of either of these bases in the last five bases of the 3' end (GC-clamp). Primers which have 1 or 2 G or C in this region are marked with a green "Yes", primers which have 0 or more than 2 G or C in this region are marked with a red "No".
More than 3 G/C out of last 5 bases at 3' end?: If more than 3 out of the last 5 bases at the 3' end are guanine or cytosine, the binding to the DNA fragment may be too strong. Primers which have less than 3 G or C in this region are marked with a green "No", primers which have 3 or more C in this region are marked with a red "Yes".
Risk of primer dimer formation in primer pair: Stretches of more than five complimentary bases between different primers used in the same PCR can lead to primer dimer formation, which can interfere with the desired hybridization and result in reduced PCR efficiency. With AMUSER the risk of inter-primer homology is assessed based on the relationship between the Tm of the primer and the complimentary bases potentially leading to undesirable dimerization. If the Tm of the potential inter-primer dimerization is at least 10C smaller than that of the annealing temperature used in the PCR cycle, the risk of homology is considered theoretical and the parameter is marked with a green "No", since most of the DNA which could undesirably hybridize is denatured at this higher temperature. If the opposite is the case, the parameter is marked with a red "Yes".
Risk of intra-primer homology (secondary structures): Similarly, stretches of more than three complimentary bases within a primer can lead to formation of intra-primer secondary structures, resulting in reduced PCR efficiency. With AMUSER the risk of intra-primer homology is assessed based on the relationship between the Tm of the primer and the complimentary bases potentially leading to undesirable intra-primer secondary structures. If the Tm of the intra-primer secondary structure formation is at least 10° C smaller than that of the annealing temperature used in the PCR cycle, the risk of homology is considered theoretical and the parameter is marked with a green "No", since most of the DNA which could undesirably hybridize is denatured at this higher temperature. If the opposite is the case, the parameter is marked with a red "Yes".
Presence of PolyN stretches: Sequences consisting of 4 or more consecutive identical nucleotides within the primer are known as polyN stretches; polyG or polyC stretches promote non-specific annealing, whereas polyA or polyT can cause the "opening" of stretches of the primer template complex, referred to as "breathing". If the primers contain stretches of more than 4 consecutive identical nucleotides within a primer, the parameter is highlighted with a red "Yes", if not; the parameter is highlighted with a red "No".
Tm of primers within 2C of each other: For a given primer pair, the primers' Tm should be within 2C of each other, in order to achieve efficient PCR amplification. If this is the case, the parameter is marked with a green "Yes", if not; the parameter is marked with a red "No".
Back to top
If you need help regarding technical issues (e.g. errors or missing results) contact Technical Support. Please include the name of the service and version (e.g. NetPhos-4.0) and the options you have selected. If the error occurs after the job has started running, please include the JOB ID (the long code that you see while the job is running).
If you have scientific questions (e.g. how the method works or how to interpret results), contact Correspondence.
Scientific and Technical problems:
USER cloning is a fast and versatile method for engineering of plasmid DNA. We have developed an easy to use web server tool that automates the design of optimal PCR primers for several distinct USER cloning-based applications. Our web server, named AMUSER (Automated DNA Modifications with USER cloning), facilitates DNA assembly and introduction of virtually any type of site-directed mutagenesis by designing optimal PCR primers for the desired genetic changes. To demonstrate the utility, we designed primers for a simultaneous two-position site-directed mutagenesis of green fluorescent protein (GFP) to yellow fluorescent protein (YFP), which in a single step reaction resulted in a 94% cloning efficiency. AMUSER also supports degenerate nucleotide primers, single insert combinatorial assembly, and flexible parameters for PCR amplification.
Hans Jasper Genee, Mads Tvillinggaard Bonde, Frederik Otzen Bagger, Jakob Berg Jespersen, Morten O. A. Sommer, Rasmus Wernerson, and Lars Rønn Olsen
Software-supported USER cloning strategies for site-directed mutagenesis and DNA assembly
Contact
Rasmus Wernersson: raz@cbs.dtu.dk
(Web)
If you need help regarding technical issues (e.g. errors or missing results) contact Technical Support. Please include the name of the service and version (e.g. NetPhos-4.0) and the options you have selected. If the error occurs after the job has started running, please include the JOB ID (the long code that you see while the job is running).
If you have scientific questions (e.g. how the method works or how to interpret results), contact Correspondence.
Correspondence:
Technical Support: